NEET-XII-Chemistry
Previous Year Paper year:2021
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #Section : A
- Qstn #1Right option for the number of tetrahedral and octahedral voids in hexagonal primitive unit cell are:
(A) 8,4
(B) 6,12
(C) 2,1
(D) 12,6digAnsr: DAns : D
- Qstn #2\( Zr( Z =40) \) and \( Hf ( Z =72) \) have similar atomic and ionic radii because of :
(A) belonging to same group
(B) diagonal relationship
(C) lanthanoid contraction
(D) having similar chemical propertiesdigAnsr: CAns : C
- Qstn #3For a reaction \( A \rightarrow B \) , enthalpy of reaction is \( -4.2\, kJ\, mol ^{-1} \) and enthalpy of activation is \( 9.6 \,k J\, mol ^{-1} \) . The correct potential energy profile for the reaction is shown in option.
(A)
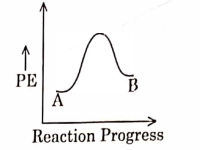
(B)
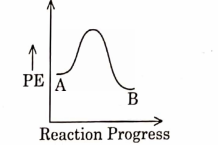
(C)
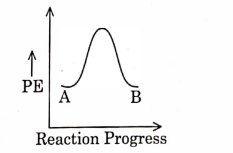
(D)
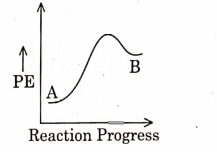 digAnsr: AAns : A
digAnsr: AAns : A
- Qstn #4Tritium, a radioactive isotope of hydrogen, emits which of the following particles?
(A) Beta \( \left(\beta^{-}\right) \)
(B) Alpha \( (\alpha) \)
(C) Gamma \( (\gamma) \)
(D) Neutron (n)digAnsr: CAns : C
- Qstn #5The \( RBC \) deficiency is deficiency disease of :
(A) Vitamin \( B_{12} \)
(B) Vitamin \( B_6 \)
(C) Vitamin \( B_1 \)
(D) Vitamin \( B_2 \)digAnsr: AAns : A
- Qstn #6The molar conductance of \( NaCl , HCl \) and \( CH _{3} COONa \) at infinite dilution are \( 126.45,426.16 \) and \( 91.0 \,S \,cm ^{2} \,mol ^{-1} \) respectively. The molar conductance of \( CH _{3} COOH \) at infinite dilution is. Choose the right option for your answer.
(A) \( 201.28 \,S \,cm ^{2} mol ^{-1} \)
(B) \( 390.71\, S\, cm ^{2} mol ^{-1} \)
(C) \( 698.28 \,S \,cm ^{2} mol ^{-1} \)
(D) \( 540.48\, S\, cm ^{2} mol ^{-1} \)digAnsr: BAns : B
- Qstn #7The correct structure of \( 2, 6 - Dimethyl-dec - 4 - ene \) is :
(A)
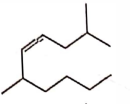
(B)
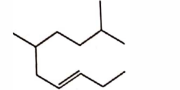
(C)
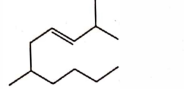
(D)
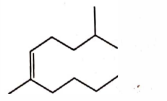 digAnsr: AAns : A
digAnsr: AAns : A
- Qstn #8The maximum temperature that can be achieved in blast furnace is :
(A) upto 1200 K
(B) upto 2200 K
(C) upto 1900 K
(D) upto 5000 KdigAnsr: BAns : B
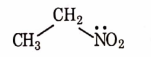
- Qstn #9Identify the compound that will react with Hinsberg’s reagent to give a solid which dissolves in alkali.
(A)
(B)
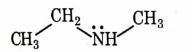
(C)
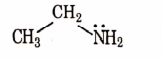
(D)
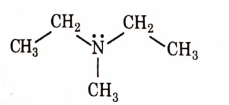 digAnsr: CAns : C
digAnsr: CAns : C
- Qstn #10The following solutions were prepared by dissolving \( 10 g \) of glucose \( \left( C _{6} H _{12} O _{6}\right) \) in \( 250 \,ml \) of water \( \left( P _{1}\right) \) \( 10 \,g \) of urea \( \left( CH _{4} N _{2} O \right) \) in \( 250 \,ml \) of water \( \left( P _{2}\right) \) and \( 10 g \) of sucrose \( \left( C _{12} H _{22} O _{11}\right) \) in \( 250\, ml \) of water \( \left( P _{3}\right) \) . The right option for the decreasing order of osmotic pressure of these solutions is:
(A) \( P _{2}> P _{1}> P _{3} \)
(B) \( P _{1}> P _{2}> P _{3} \)
(C) \( P _{2}> P _{3}> P _{1} \)
(D) \( P _{3}> P _{1}> P _{2} \)digAnsr: AAns : A
- Qstn #11The major product of the following chemical reaction is :
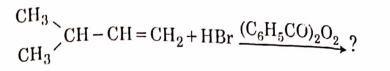
(A)
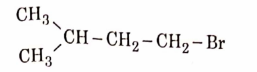
(B)
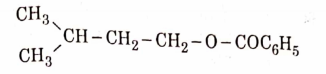
(C)
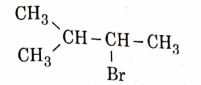
(D)
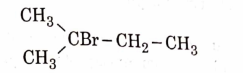 digAnsr: AAns : A
digAnsr: AAns : A
- Qstn #12Given below are two statements : Statement I : Aspirin and Paracetamol belong to the class of narcotic analgesics. Statement II : Morphine and Heroin are non-narcotic analgesics. In the light of the above statements, choose the correct answer from the options given below.
(A) Both Statement I and Statement II are true.
(B) Both Statement I and Statement II are false.
(C) Statement I is correct but Statement II is false.
(D) Statement I is incorrect but Statement II is true.digAnsr: AAns : A
- Qstn #13The correct sequence of bond enthalpy of ' \( C - X \) ' bond is:
(A) \( CH _{3}- F < CH _{3}- Cl < CH _{3}- Br < CH _{3}- I \)
(B) \( CH _{3}- F > CH _{3}- Cl > CH _{3}- Br > CH _{3}- I \)
(C) \( CH _{3}- F < CH _{3}- Cl > CH _{3}- Br > CH _{3}- I \)
(D) \( CH _{3}- Cl > CH _{3}- F > CH _{3}- Br > CH _{3}- I \)digAnsr: BAns : B