NEET-XII-Chemistry
Previous Year Paper year:2018
- #56Consider the change in oxidation state of Bromine
corresponding to different emf values as shown in
the diagram below:
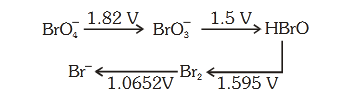
Then the species undergoing disproportionation is:-
(1) ``\ce{BrO3-}``
(2) ``\ce{BrO4-}``
(3) ``\ce{Br2}``
(4) HBrOdigAnsr: 4Ans : (4)
Sol. Calculate E°cell corresponding to each compound
under going disproportionation reaction. The
reaction for which E°cell comes out +ve is
spontaneous.
HBrO Br2 E° = 1.595, SRP (cathode)
HBrO BrO3
- E° = -1.5V, SOP (Anode)
2HBrO Br2 + BrO3
-
E°cell = SRP (cathode) - SRP (Anode)
= 1.595 - 1.5
= 0.095 V
E°cell > 0 &implies; ▵G° < 0 [spontaneous]