NEET-XII-Chemistry
04: Amines
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- Qstn #4-iiiCH3NH2, (CH3)2NH, (CH3)3N, C6H5NH2, C6H5CH2NH2.


Ans : Considering the inductive effect and the steric hindrance of alkyl groups, CH3NH2, (CH3)2NH, and (CH3)3N can be arranged in the increasing order of their basic strengths as:
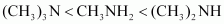
In C6H5NH2, N is directly attached to the benzene ring. Thus, the lone pair of electrons on the N-atom is delocalized over the benzene ring. In C6H5CH2NH2, N is not directly attached to the benzene ring. Thus, its lone pair is not delocalized over the benzene ring. Therefore, the electrons on the N atom are more easily available for protonation in C6H5CH2NH2 than in C6H5NH2 i.e., C6H5CH2NH2 is more basic than C6H5NH2.
Again, due to the -I effect of C6H5 group, the electron density on the N-atom in C6H5CH2NH2 is lower than that on the N-atom in (CH3)3N. Therefore, (CH3)3N is more basic than C6H5CH2NH2. Thus, the given compounds can be arranged in the increasing order of their basic strengths as follows.

- Qstn #6Write reactions of the final alkylation product of aniline with excess of methyl
iodide in the presence of sodium carbonate solution.
Ans : Aniline reacts with methyl iodide to produce N, N-dimethylaniline.
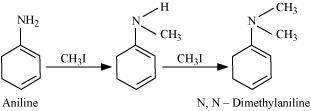
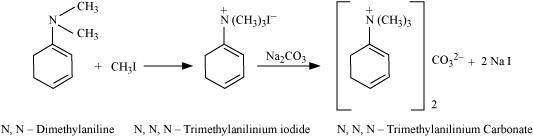
With excess methyl iodide, in the presence of Na2CO3 solution, N, N-dimethylaniline produces N, N, N-trimethylanilinium carbonate.
- Qstn #7Write chemical reaction of aniline with benzoyl chloride and write the name of
the product obtained.
Ans :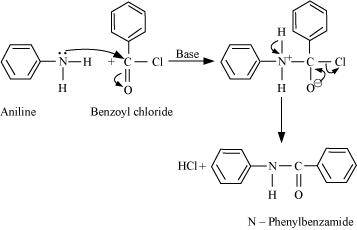
- Qstn #8Write structures of different isomers corresponding to the molecular formula,
C3H9N. Write IUPAC names of the isomers which will liberate nitrogen gas on
treatment with nitrous acid.
Ans : The structures of different isomers corresponding to the molecular formula, C3H9N are given below:
(a)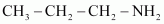
Propan-1-amine (10)
(b)
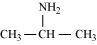
Propan-2-amine (10)
(c)

(d)
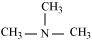
N,N-Dimethylmethanamine (30)
10amines, (a) propan-1-amine, and (b) Propan-2-amine will liberate nitrogen gas on treatment with nitrous acid.
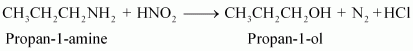

SECTION I SECTION I Page No 399:
- #Section : IISECTION I Page No 400:
- Qstn #1Write IUPAC names of the following compounds and classify them into primary,
secondary and tertiary amines.