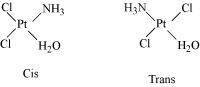NEET-XII-Chemistry
09: Coordination Compounds
- #3Indicate the types of isomerism exhibited by the following complexes and
draw the structures for these isomers:
- K[Cr(H2O)2(C2O4)2
- [Co(en)3]Cl3
- [Co(NH3)5(NO2)](NO3)2
- [Pt(NH3)(H2O)Cl2]
Ans :- Both geometrical (cis-, trans-) isomers for
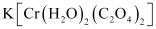 can exist. Also, optical isomers for cis-isomer exist.
can exist. Also, optical isomers for cis-isomer exist.
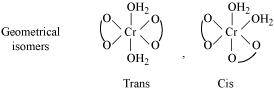
Trans-isomer is optically inactive. On the other hand, cis-isomer is optically active.
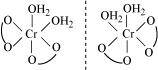
(ii) Two optical isomers for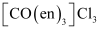 exist.
exist.
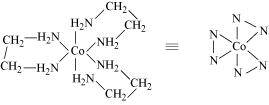
Two optical isomers are possible for this structure.

(iii)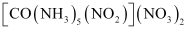
A pair of optical isomers:
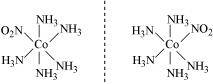
It can also show linkage isomerism.
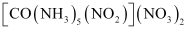 and
and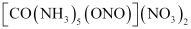
It can also show ionization isomerism.
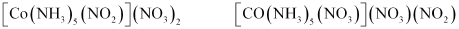
(iv) Geometrical (cis-, trans-) isomers of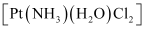 can exist.
can exist.