NEET-XII-Chemistry
09: Coordination Compounds
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
09-Coordination Compounds
Note: Please signup/signin free to get personalized experience.
- Qstn #3-iiligand,Ans : Ligands
The neutral molecules or negatively charged ions that surround the metal atom in a coordination entity or a coordinal complex are known as ligands. For example,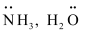 , Cl-, -OH. Ligands are usually polar in nature and possess at least one unshared pair of valence electrons.
, Cl-, -OH. Ligands are usually polar in nature and possess at least one unshared pair of valence electrons.
- Qstn #3-iiicoordination number,Ans : Coordination number:
The total number of ligands (either neutral molecules or negative ions) that get attached to the central metal atom in the coordination sphere is called the coordination number of the central metal atom. It is also referred to as its ligancy.
For example:
(a) In the complex, K2[PtCl6], there as six chloride ions attached to Pt in the coordinate sphere. Therefore, the coordination number of Pt is 6.
(b) Similarly, in the complex [Ni(NH3)4]Cl2, the coordination number of the central atom (Ni) is 4.
- Qstn #3-ivcoordination polyhedron,Ans : Coordination polyhedron:
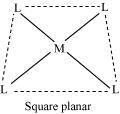
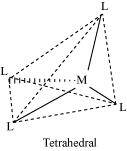
Coordination polyhedrons about the central atom can be defined as the spatial arrangement of the ligands that are directly attached to the central metal ion in the coordination sphere. For example:
(a)
(b) Tetrahedral
- Qstn #3-vhomoleptic andAns : Homoleptic complexes:
These are those complexes in which the metal ion is bound to only one kind of a donor group. For eg: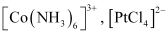 etc.
etc.
- Qstn #3-viheteroleptic.Ans : Heteroleptic complexes:
Heteroleptic complexes are those complexes where the central metal ion is bound to more than one type of a donor group.
For e.g.: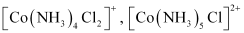
- Qstn #4What is meant by unidentate, didentate and ambidentate ligands? Give two examples for each.
Ans : A ligand may contain one or more unshared pairs of electrons which are called the donor sites of ligands. Now, depending on the number of these donor sites, ligands can be classified as follows:
(a) Unidentate ligands: Ligands with only one donor sites are called unidentate ligands. For e.g., , Cl- etc.
, Cl- etc.
(b) Didentate ligands: Ligands that have two donor sites are called didentate ligands. For e.g.,
(a) Ethane-1,2-diamine
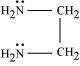
(b) Oxalate ion
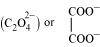
(c) Ambidentate ligands:
Ligands that can attach themselves to the central metal atom through two different atoms are called ambidentate ligands. For example:
(a)
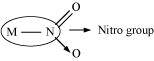
(The donor atom is N)
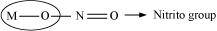
(The donor atom is oxygen)
(b)

(The donor atom is S)

(The donor atom is N)
- Qstn #5-iinullnullnullnull[CoBr2(en)2]+
(null) [PtCl4]2-
(null) K3[Fe(CN)6]
(null) ``\ce{Cr(NH3)Cl3}`` () [Co(H2O)(CN)(en)2]2+Ans :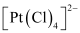
Let the oxidation number of Pt be x.
The charge on the complex is -2.
x + 4(-1) = -2
x = + 2
(null) (iii)
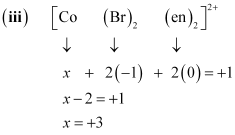
(null) (iv)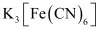
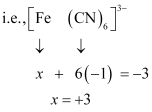
(null) (v)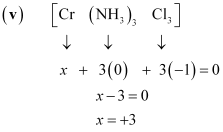 ()
() 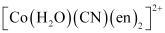
Let the oxidation number of Co be x.
The charge on the complex is +2.
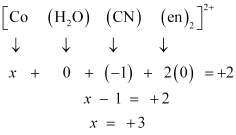
- Qstn #5-i[Co(H2O)(CN)(en)2]2+Ans :
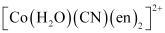
Let the oxidation number of Co be x.
The charge on the complex is +2.
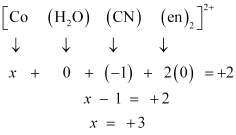
- Qstn #6-iTetrahydroxozincate(II)
() Potassium tetrachloridopalladate(II)
() Diamminedichloridoplatinum(II)
() Potassium tetracyanonickelate(II)
() Tetrabromidocuprate(II)
() Pentaamminenitrito-O-cobalt(III)
() Hexaamminecobalt(III) sulphate
() Potassium tri(oxalato)chromate(III)
() Hexaammineplatinum(IV)
() Pentaamminenitrito-N-cobalt(III)Ans : [Zn(OH)4]2-
() K2[PdCl4]
() [Pt(NH3)2Cl2]
() K2[Ni(CN)4]
() [Cu(Br)4]2-
() [Co(ONO) (NH3)5]2+
() [Co(NH3)6]2 (SO4)3
() K3[Cr(C2O4)3]
() [Pt(NH3)6]4+
() [Co[NO2)(NH3)5]2+