NEET-XII-Chemistry
09: Coordination Compounds
- #7[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3- is weakly paramagnetic. Explain.
Ans : In both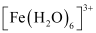 and
and  , Fe exists in the +3 oxidation state i.e., in d5 configuration.
, Fe exists in the +3 oxidation state i.e., in d5 configuration.

Since CN- is a strong field ligand, it causes the pairing of unpaired electrons. Therefore, there is only one unpaired electron left in the d-orbital.

Therefore,
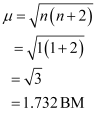
On the other hand, H2O is a weak field ligand. Therefore, it cannot cause the pairing of electrons. This means that the number of unpaired electrons is 5.
Therefore,
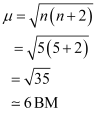
Thus, it is evident that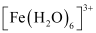 is strongly paramagnetic, while
is strongly paramagnetic, while  is weakly paramagnetic.
is weakly paramagnetic.