NEET-XII-Chemistry
09: Coordination Compounds
- #5Explain on the basis of valence bond theory that [Ni(CN)4]2- ion with square
planar structure is diamagnetic and the [NiCl4]2- ion with tetrahedral geometry is paramagnetic.
Ans : Ni is in the +2 oxidation state i.e., in d8 configuration.

There are 4 CN- ions. Thus, it can either have a tetrahedral geometry or square planar geometry. Since CN- ion is a strong field ligand, it causes the pairing of unpaired 3d electrons.
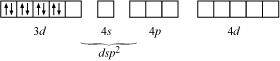
It now undergoes dsp2 hybridization. Since all electrons are paired, it is diamagnetic.
In case of [NiCl4]2-, Cl- ion is a weak field ligand. Therefore, it does not lead to the pairing of unpaired 3d electrons. Therefore, it undergoes sp3 hybridization.
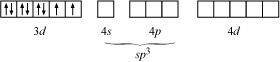
Since there are 2 unpaired electrons in this case, it is paramagnetic in nature.