NEET-XI-Chemistry
03: The s-Block Elements
- #12Discuss the various reactions that occur in the Solvay process.
Ans : Solvay process is used to prepare sodium carbonate.
When carbon dioxide gas is bubbled through a brine solution saturated with ammonia, sodium hydrogen carbonate is formed. This sodium hydrogen carbonate is then converted to sodium carbonate.
Step 1: Brine solution is saturated with ammonia.
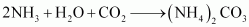
This ammoniated brine is filtered to remove any impurity.
Step 2: Carbon dioxide is reacted with this ammoniated brine to result in the formation of insoluble sodium hydrogen carbonate.
NH3+H2O+CO2→NH4HCO3NaCl+NH4HCO3→NaHCO3+NH4Cl
Step 3: The solution containing crystals of NaHCO3 is filtered to obtain NaHCO3.
Step 4: NaHCO3 is heated strongly to convert it into NaHCO3.
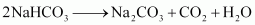
Step 5: To recover ammonia, the filtrate (after removing NaHCO3) is mixed with Ca(OH)2 and heated.
Ca(OH)2+2NH4Cl→2NH3+2H2O+CaCl2
The overall reaction taking place in Solvay process is