NEET-XI-Chemistry
03: The s-Block Elements
- #10When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reasons for this type of colour change.
Ans : When an alkali metal is dissolved in liquid ammonia, it results in the formation of a deep blue coloured solution.
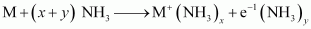
The ammoniated electrons absorb energy corresponding to red region of visible light. Therefore, the transmitted light is blue in colour.
At a higher concentration (3 M), clusters of metal ions are formed. This causes the solution to attain a copper-bronze colour and a characteristic metallic lustre.