NEET-XI-Chemistry
02: Hydrogen
- #10Do you expect the carbon hydrides of the type (CnH2n + 2) to act as ‘Lewis’ acid or base? Justify your answer.
Ans : For carbon hydrides of type CnH2n + 2, the following hydrides are possible for
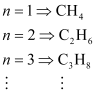
For a hydride to act as a Lewis acid i.e., electron accepting, it should be electron-deficient. Also, for it to act as a Lewis base i.e., electron donating, it should be electron-rich.
Taking C2H6 as an example, the total number of electrons are 14 and the total covalent bonds are seven. Hence, the bonds are regular 2e--2 centered bonds.
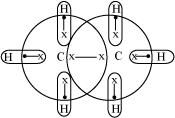
Hence, hydride C2H6 has sufficient electrons to be represented by a conventional Lewis structure. Therefore, it is an electron-precise hydride, having all atoms with complete octets. Thus, it can neither donate nor accept electrons to act as a Lewis acid or Lewis base.