NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #12H3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same.
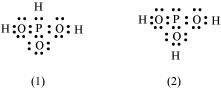
Ans : The given structures cannot be taken as the canonical forms of the resonance hybrid of H3PO3 because the positions of the atoms have changed.