NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #7Discuss the shape of the following molecules using the VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Ans : BeCl2:
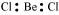
The central atom has no lone pair and there are two bond pairs. i.e., BeCl2 is of the type AB2. Hence, it has a linear shape.
BCl3:
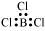
The central atom has no lone pair and there are three bond pairs. Hence, it is of the type AB3. Hence, it is trigonal planar.
SiCl4:
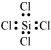
The central atom has no lone pair and there are four bond pairs. Hence, the shape of SiCl4 is tetrahedral being the AB4 type molecule.
AsF5:
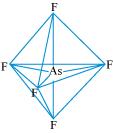
The central atom has no lone pair and there are five bond pairs. Hence, AsF5 is of the type AB5. Therefore, the shape is trigonal bipyramidal.
H2S:
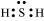
The central atom has one lone pair and there are two bond pairs. Hence, H2S is of the type AB2E. The shape is Bent.
PH3:
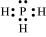
The central atom has one lone pair and there are three bond pairs. Hence, PH3 is of the AB3E type. Therefore, the shape is trigonal pyramidal.