ICSE-X-Chemistry
Previous Year Paper year:2014
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #1-b-iThe basicity of acetic acid is-------- (3, 1, 4).
- #1-b-iiThe compound formed when ethanol reacts with sodium is--------- (sodium ethanoate,
sodium ethoxide, sodium propanoate).
- #1-b-iiiQuicklime is not used to dry ``\ce{HCl}`` gas because--------- (``\ce{CaO}`` is alkaline, ``\ce{CaO}`` is acidic, ``\ce{CaO}`` is neutral).
- #1-b-ivAmmonia gas is collected by---------- (an upward displacement of air, a downward
displacement of water, a downward displacement of air).
- #1-b-v [5]Cold, dilute nitric acid reacts with copper to form--------- (hydrogen, nitrogen dioxide,
nitric oxide).
- #1-cGive one word or phrase for the following:
- #1-c-iThe ratio of the mass of a certain volume of gas to the mass of an equal volume of
hydrogen under the same conditions of temperature and pressure
- #1-c-iiFormation of ions from molecules
- #1-c-iiiElectrolytic deposition of a superior metal on a baser metal
- #1-c-ivHydrocarbons containing a
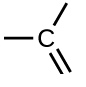
functional group
- #1-c-v [5]The amount of energy released when an atom in the gaseous state accepts an electron
to form an anion
- #1-d [5]Match the options A to E with the statements (i) to (v):
A. Alkyne (i) No. of molecules in ``\pu{22.4 dm-3}`` of carbon
dioxide at STPB. Alkane (ii) An element with electronic configuration 2,8,8,3 C. Iron (iii) ``\ce{C_nH_{2n+2}}`` D. ``\pu{6.023E23}`` (iv) ``\ce{C_nH_{2n-2}}`` E. Metal (v) The metal which forms two types of ions
- #1-eWrite balanced equations for the following:
- #1-e-iAction of heat on a mixture of copper and concentrated nitric acid
- #1-e-iiAction of warm water on magnesium nitride