CBSE-XI-Physics
24: Kinetic Theory of Gases
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #12If the molecules were not allowed to collide among themselves, would you expect more evaporation or less evaporation?Ans : If the molecules are not allowed to collide with each other, they will have long mean free paths and hence, evaporation will be faster. In vacuum, the external pressure will be very low. So, the liquid will boil and evaporate at very low temperature.
- Qstn #13Is it possible to boil water at room temperature, say 30°C? If we touch a flask containing water boiling at this temperature, will it be hot?Ans : Yes, it is possible to boil water at 300C by reducing the external pressure. A liquid boils when its vapour pressure equals external pressure. By lowering the external pressure, it is possible to boil the liquid at low temperatures.
No, the flask containing water boiling at 300C will not be hot.
- Qstn #14When you come out of a river after a dip, you feel cold. Explain.Ans : After a dip in the river, the water that sticks to our body gets evaporated. We know that evaporation takes place faster for higher temperatures. Thus, the molecules that have the highest kinetic energy leave faster and that is how heat is given away from our body.
As a result of it, temperature of our body falls down due to loss of heat and we feel cold.
- #Section : ii
- Qstn #1Which of the following parameters is the same for molecules of all gases at a given temperature?
(a) Mass
(b) Speed
(c) Momentum
(d) Kinetic energy.digAnsr: dAns : (d) Kinetic energy.
Temperature is defined as the average kinetic energy of the molecules in a gas sample. Average is same for all the molecules of the sample. So, kinetic energy is the same for all.
Hence, correct answer is
(d).
- Qstn #2A gas behaves more closely as an ideal gas at
(a) low pressure and low temperature
(b) low pressure and high temperature
(c) high pressure and low temperature
(d) high pressure and high temperature.digAnsr: bAns : At low pressure, the concentration of gas molecules is very low. Hence, the kinetic assumption that the size of the molecules can be neglected compared to the volume of the container applies.
At high temperature, molecules move very fast. So, they tend to collide elastically and forces of interaction between the molecules minimise. This is the required idea condition.
Thus,
(b) is the correct answer.
- Qstn #3The pressure of an ideal gas is written as
P=2E3V. Here E refers to
(a) translational kinetic energy
(b) rotational kinetic energy
(c) vibrational kinetic energy
(d) total kinetic energy.digAnsr: aAns : According to the kinetic theory, molecules show straight line in motion (translational). So, the kinetic energy is essentially transitional.
Thus,
(a) is the correct answer.
- Qstn #4The energy of a given sample of an ideal gas depends only on its
(a) volume
(b) pressure
(c) density
(d) temperature.digAnsr: dAns : Temperature of a gas is directly proportional to its kinetic energy. Thus, energy of an ideal gas depends only on its temperature.
Thus,
(d) is the correct answer.
- Qstn #5Which of the following gases has maximum rms speed at a given temperature?
(a) hydrogen
(b) nitrogen
(c) oxygen
(d) carbon dioxide.digAnsr: aAns : The rms speed of a gas is given by `` \sqrt{\frac{3RT}{{M}_{o}}}``.
Since hydrogen has the lowest Mo compared to other molecules, it will have the highest rms speed.
Thus,
(a) is the correct answer.
- Qstn #6Figure shows graphs of pressure vs density for an ideal gas at two temperatures T1 and T2.
(a) T1 > T2
(b) T1 = T2
(c) T1 < T2
(d) Any of the three is possible.
Figure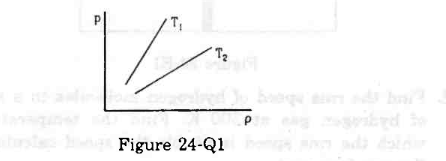 digAnsr: aAns : The straight line T1 has greater slope than T2. This means `` \frac{P}{\rho }`` ratio is greater for T1 than T2. Now, rms velocity of a gas is given by `` \sqrt{\frac{3P}{\rho }}``. This means rms velocity of gas with T1 molecules is greater than T2 molecules. Again, gas with higher temperature has higher rms velocity.
digAnsr: aAns : The straight line T1 has greater slope than T2. This means `` \frac{P}{\rho }`` ratio is greater for T1 than T2. Now, rms velocity of a gas is given by `` \sqrt{\frac{3P}{\rho }}``. This means rms velocity of gas with T1 molecules is greater than T2 molecules. Again, gas with higher temperature has higher rms velocity.
So, T1 > T2.
Thus,
(a) is the correct answer.
- Qstn #7The mean square speed of the molecules of a gas at absolute temperature T is proportional to
(a)
1T
(b)
T
(c) T
(d) T2.digAnsr: cAns : Root mean squared velocity is given by
`` {v}_{rms}=\sqrt{\frac{3RT}{M}}``
`` \Rightarrow {\left({v}_{rms}\right)}^{2}=\frac{3RT}{M}``
`` \Rightarrow {\left({v}_{rms}\right)}^{2}\alpha T``
Thus,
(c) is the correct answer.
- Qstn #8Suppose a container is evacuated to leave just one molecule of a gas in it. Let va and vrms represent the average speed and the rms speed of the gas.
(a) va > vrms
(b) va < vrms
(c) va = vrms
(d) vrms is undefined.digAnsr: cAns : Speed is constant and same for a single molecule. Thus, rms speed will be equal to its average speed.
Thus,
(c) is the correct answer.
- Qstn #9The rms speed of oxygen at room temperature is about 500 m/s. The rms speed of hydrogen at the same temperature is about
(a) 125 m s-1
(b) 2000 m s-1
(c) 8000 m s-1
(d) 31 m s-1.digAnsr: bAns : (b) 2000 ms-1
Given,
Molecular mass of hydrogen, MH = 2
Molecular mass of oxygen, Mo = 32
RMS speed is given by,
`` {v}_{rms=}\sqrt{\frac{3RT}{M}}``
`` ``
`` \Rightarrow \sqrt{\frac{\mathit{3}RT}{{M}_{O}}}=500``
`` ``
`` ``
`` ``
`` ``
`` ``
Now,
`` ``
`` \Rightarrow \frac{{v}_{Orms}}{{v}_{Hrms}}=\frac{\sqrt{{\displaystyle \frac{3RT}{{M}_{O}}}}}{\sqrt{{\displaystyle \frac{3RT}{{M}_{H}}}}}``
`` \Rightarrow \frac{{v}_{O}rms}{{v}_{Hrms}}=\frac{\sqrt{{\displaystyle \frac{3RT}{32}}}}{\sqrt{{\displaystyle \frac{3RT}{2}}}}``
`` \Rightarrow \frac{{v}_{Orms}}{{{v}_{H}}_{rms}}=\frac{1}{4}``
`` \Rightarrow \frac{500}{{v}_{Hrms}}=\frac{1}{4}``
`` \Rightarrow {v}_{Hrms}=4\times 500=2000{\,\mathrm{\,ms\,}}^{-1}``
`` ``
`` ``
`` ``
Hence, the correct answer is
(b).
- Qstn #10The pressure of a gas kept in an isothermal container is 200 kPa. If half the gas is removed from it, the pressure will be
(a) 100 kPa
(b) 200 kPa
(c) 400 kPa
(d) 800 kPa.digAnsr: aAns : Let the number of moles in the gas be n.
Applying equation of state, we get
`` PV=nRT``
`` \Rightarrow P=\frac{nRT}{V}``
`` \Rightarrow 2\times {10}^{5}=\frac{nRT}{V}...\left(1\right)``
`` \,\mathrm{\,When\,}\,\mathrm{\,half\,}\,\mathrm{\,of\,}\,\mathrm{\,the\,}\,\mathrm{\,gas\,}\,\mathrm{\,is\,}\,\mathrm{\,removed\,},\,\mathrm{\,number\,}\,\mathrm{\,of\,}\,\mathrm{\,moles\,}\,\mathrm{\,left\,}\,\mathrm{\,behind\,}=\frac{\,\mathrm{\,n\,}}{2}``
`` \,\mathrm{\,Suppose\,}\,\mathrm{\,the\,}\,\mathrm{\,pressure\,}\,\mathrm{\,be\,}\,\mathrm{\,P\,}\text{'}.``
`` \,\mathrm{\,P\,}\text{'}=\frac{\,\mathrm{\,n\,}}{2}\frac{\,\mathrm{\,R\,}\,\mathrm{\,T\,}}{\,\mathrm{\,V\,}}``
`` \,\mathrm{\,Now\,},``
`` \,\mathrm{\,P\,}\text{'}=\frac{1}{2}\times 2\times {10}^{5}={10}^{5}\left[\,\mathrm{\,From\,}\,\mathrm{\,eq\,}.\left(1\right)\right]``
`` ``
`` ``
`` ``
=100 kPa
Thus,
(a) is the correct answer.
- Qstn #11The rms speed of oxygen molecules in a gas is v. If the temperature is doubled and the oxygen molecules dissociate into oxygen atoms, the rms speed will become
(a) v
(b)
v2
(c) 2v
(d) 4v.digAnsr: cAns : `` \,\mathrm{\,Given\,}:\,\mathrm{\,v\,}=\sqrt{\frac{3\,\mathrm{\,RT\,}}{32}}``
`` \,\mathrm{\,Suppose\,}\,\mathrm{\,the\,}\,\mathrm{\,new\,}\,\mathrm{\,rms\,}\,\mathrm{\,speed\,}\,\mathrm{\,be\,}\,\mathrm{\,v\,}\text{'}.``
`` \,\mathrm{\,Molecule\,}\,\mathrm{\,dissociate\,},M=16``
`` ``
`` v\text{'}=\sqrt{\frac{3R\left(2T\right)}{16}}``
`` =\sqrt{\frac{3R\left(4T\right)}{32}}``
`` =2\sqrt{\frac{3RT}{32}}=2v``
Thus,
(c) is the correct answer.