NEET-XII-Chemistry
01: Haloalkanes and Haloarenes
- #2Why is sulphuric acid not used during the reaction of alcohols with KI?
Ans : In the presence of sulphuric acid (H2SO4), KI produces HI

Since
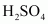 is an oxidizing agent, it oxidizes HI (produced in the reaction to I2).
is an oxidizing agent, it oxidizes HI (produced in the reaction to I2).

As a result, the reaction between alcohol and HI to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidizing acid such as H3PO4 is used.