Loading…
NEET-XII-Chemistry
04: Amines
- Qstn #2-iMethylamine and dimethylamineAns : Methylamine and dimethylamine can be distinguished by the carbylamine test.
Carbylamine test: Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form foul-smelling isocyanides or carbylamines. Methylamine (being an aliphatic primary amine) gives a positive carbylamine test, but dimethylamine does not.
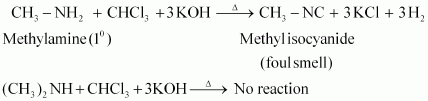
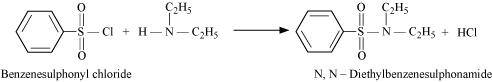
- Qstn #2-iiSecondary and tertiary aminesAns : Secondary and tertiary amines can be distinguished by allowing them to react with Hinsberg’s reagent (benzenesulphonyl chloride, C6H5SO2Cl).
Secondary amines react with Hinsberg’s reagent to form a product that is insoluble in an alkali. For example, N, N-diethylamine reacts with Hinsberg’s reagent to form N, N-diethylbenzenesulphonamide, which is insoluble in an alkali. Tertiary amines, however, do not react with Hinsberg’s reagent.
- Qstn #2-iiiEthylamine and anilineAns : Ethylamine and aniline can be distinguished using the azo-dye test. A dye is obtained when aromatic amines react with HNO2 (NaNO2 + dil.HCl) at 0-5°C, followed by a reaction with the alkaline solution of 2-naphthol. The dye is usually yellow, red, or orange in colour. Aliphatic amines give a brisk effervescence due (to the evolution of N2 gas) under similar conditions.
(2).png) ​
​
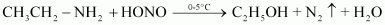
- Qstn #2-ivAniline and benzylamineAns : Aniline and benzylamine can be distinguished by their reactions with the help of nitrous acid, which is prepared in situ from a mineral acid and sodium nitrite. Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
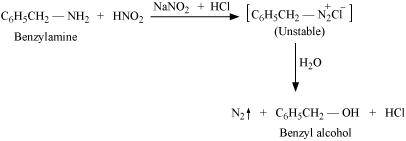
On the other hand, aniline reacts with HNO2 at a low temperature to form stable diazonium salt. Thus, nitrogen gas is not evolved.
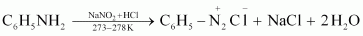
- Qstn #2-vAniline and N-methylaniline.Ans : Aniline and N-methylaniline can be distinguished using the Carbylamine test. Primary amines, on heating with chloroform and ethanolic potassium hydroxide, form foul-smelling isocyanides or carbylamines. Aniline, being an aromatic primary amine, gives positive carbylamine test. However, N-methylaniline, being a secondary amine does not.
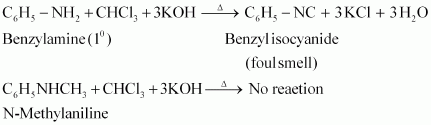
- Qstn #3-ipKb of aniline is more than that of methylamine.Ans : pKb of aniline is more than that of methylamine:
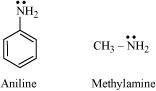
Aniline undergoes resonance and as a result, the electrons on the N-atom are delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate.

On the other hand, in case of methylamine (due to the +I effect of methyl group), the electron density on the N-atom is increased. As a result, aniline is less basic than methylamine. Thus, pKb of aniline is more than that of methylamine.
- Qstn #3-iiEthylamine is soluble in water whereas aniline is not.Ans : Ethylamine is soluble in water whereas aniline is not:
Ethylamine when added to water forms intermolecular H-bonds with water. Hence, it is soluble in water.
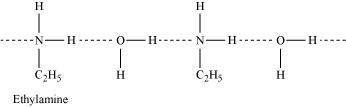
But aniline does not undergo H-bonding with water to a very large extent due to the presence of a large hydrophobic -C6H5 group. Hence, aniline is insoluble in water.
- Qstn #3-iiiMethylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.Ans : Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide:
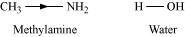
Due to the +I effect of -CH3 group, methylamine is more basic than water. Therefore, in water, methylamine produces OH- ions by accepting H+ ions from water.
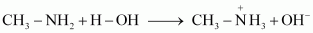
Ferric chloride (FeCl3) dissociates in water to form Fe3+ and Cl- ions.
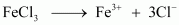
Then, OH- ion reacts with Fe3+ ion to form a precipitate of hydrated ferric oxide.
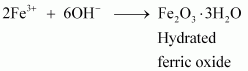
- Qstn #3-ivAlthough amino group is o, p- directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.Ans : Although amino group is o,p- directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline:
Nitration is carried out in an acidic medium. In an acidic medium, aniline is protonated to give anilinium ion (which is meta-directing).
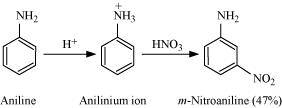
For this reason, aniline on nitration gives a substantial amount of m-nitroaniline.