NEET-XII-Chemistry
03: Aldehydes Ketones and Carboxylic Acids
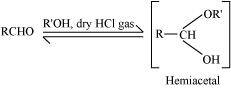
- Qstn #1-vHemiacetalAns : Hemiacetal:
Hemiacetals are α-alkoxyalcohols
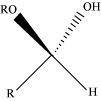
General structure of a hemiacetal
Aldehyde reacts with one molecule of a monohydric alcohol in the presence of dry HCl gas.
- Qstn #1-viOximeAns : Oxime:
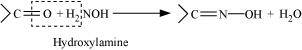
Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime.
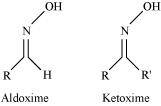
On treatment with hydroxylamine in a weakly acidic medium, aldehydes or ketones form oximes.
- Qstn #1-viiKetalAns : Ketal:
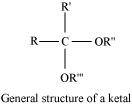
Ketals are gem-dialkoxyalkanes in which two alkoxy groups are present on the same carbon atom within the chain. The other two bonds of the carbon atom are connected to two alkyl groups.
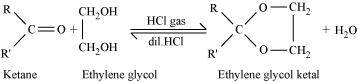
Ketones react with ethylene glycol in the presence of dry HCl gas to give a cyclic product known as ethylene glycol ketals.
- Qstn #1-viiiImineAns : Imine:
Imines are chemical compounds containing a carbon nitrogen double bond.
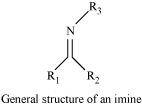
Imines are produced when aldehydes and ketones react with ammonia and its derivatives.
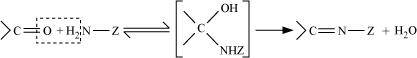
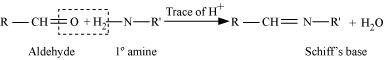
- Qstn #1-xSchiff’s baseAns : Schiff’s base:
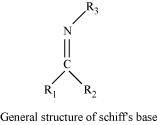
Schiff’s base (or azomethine) is a chemical compound containing a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group-but not hydrogen. They have the general formula R1R2C = NR3. Hence, it is an imine.
It is named after a scientist, Hugo Schiff.
Aldehydes and ketones on treatment with primary aliphatic or aromatic amines in the presence of trace of an acid yields a Schiff’s base.