NEET-XII-Chemistry
09: Coordination Compounds
- #9How many geometrical isomers are possible in the following coordination entities?
() [Cr(C2O4)3]3- (ii) [Co(NH3)3Cl3]
() [Cr(C2O4)3]3- (ii) [Co(NH3)3Cl3]
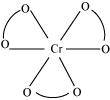
() [Cr(C2O4)3]3- (ii) [Co(NH3)3Cl3]Ans : null () For [Cr(C2O4)3]3-, no geometric isomer is possible as it is a bidentate ligand.
(ii) [Co(NH3)3Cl3]
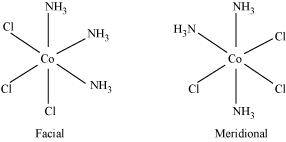
Two geometrical isomers are possible.
() For [Cr(C2O4)3]3-, no geometric isomer is possible as it is a bidentate ligand.

(ii) [Co(NH3)3Cl3]
Two geometrical isomers are possible.

() For [Cr(C2O4)3]3-, no geometric isomer is possible as it is a bidentate ligand.

(ii) [Co(NH3)3Cl3]
Two geometrical isomers are possible.

- #9-i[Cr(C2O4)3]3- (ii) [Co(NH3)3Cl3]Ans : For [Cr(C2O4)3]3-, no geometric isomer is possible as it is a bidentate ligand.

(ii) [Co(NH3)3Cl3]
Two geometrical isomers are possible.
