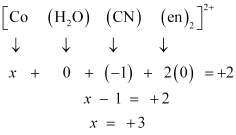NEET-XII-Chemistry
09: Coordination Compounds
- #5-iinullnullnullnullnullnullnull[CoBr2(en)2]+
(null) [PtCl4]2-
(null) K3[Fe(CN)6]
(null) ``\ce{Cr(NH3)Cl3}`` () [Co(H2O)(CN)(en)2]2+
(null) [PtCl4]2-
(null) K3[Fe(CN)6]
(null) ``\ce{Cr(NH3)Cl3}`` () [Co(H2O)(CN)(en)2]2+Ans :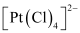
Let the oxidation number of Pt be x.
The charge on the complex is -2.
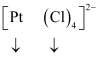
x + 4(-1) = -2
x = + 2
(null) (iii)
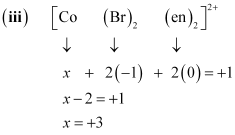
(null) (iv)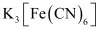
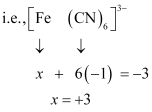
(null) (v)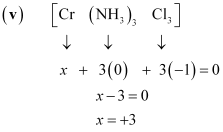 ()
() 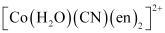
Let the oxidation number of Co be x.
The charge on the complex is +2.
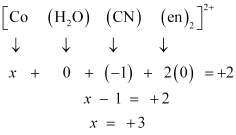
(null) (iii)
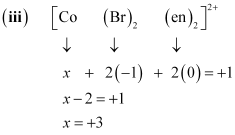
(null) (iv)
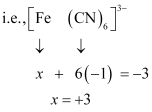
(null) (v)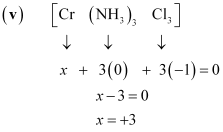 ()
() 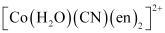
Let the oxidation number of Co be x.
The charge on the complex is +2.
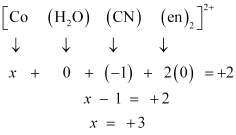
- #5-iii[PtCl4]2-Ans : (iii)
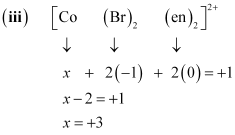
- #5-ivK3[Fe(CN)6]Ans : (iv)
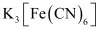
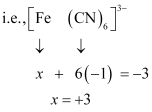
- #5-v``\ce{Cr(NH3)Cl3}``Ans : (v)
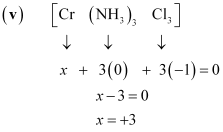
- #5-i[Co(H2O)(CN)(en)2]2+Ans :
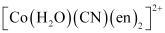
Let the oxidation number of Co be x.
The charge on the complex is +2.