NEET-XII-Chemistry
07: The p-Block Elements
- #38Give the formula and describe the structure of a noble gas species which is isostructural with:
()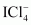
()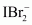
()
()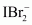
()
()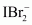
()
()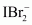
() Ans : null () XeF4 is isoelectronic with
Ans : null () XeF4 is isoelectronic with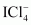 and has square planar geometry.
and has square planar geometry.
() XeF2 is isoelectronic to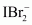 and has a linear structure.
and has a linear structure.
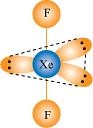
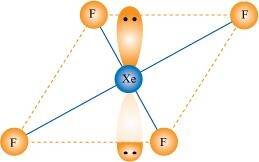
() XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.
() XeF2 is isoelectronic to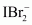 and has a linear structure.
and has a linear structure.

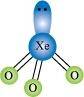
() XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.

() XeF2 is isoelectronic to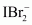 and has a linear structure.
and has a linear structure.

() XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.

() XeF2 is isoelectronic to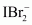 and has a linear structure.
and has a linear structure.

() XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.

- #38-i
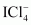
()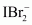
()
()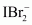
()
()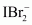
() Ans : XeF4 is isoelectronic with
Ans : XeF4 is isoelectronic with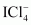 and has square planar geometry.
and has square planar geometry.

() XeF2 is isoelectronic to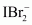 and has a linear structure.
and has a linear structure.

() XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.

() XeF2 is isoelectronic to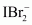 and has a linear structure.
and has a linear structure.

() XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.

() XeF2 is isoelectronic to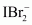 and has a linear structure.
and has a linear structure.

() XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.

- #38-ii
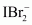 Ans : XeF2 is isoelectronic to
Ans : XeF2 is isoelectronic to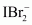 and has a linear structure.
and has a linear structure.

- #38-iii
 Ans : XeO3 is isostructural to
Ans : XeO3 is isostructural to and has a pyramidal molecular structure.
and has a pyramidal molecular structure.
