NEET-XII-Chemistry
07: The p-Block Elements
- #21Describe the manufacture of H2SO4 by contact process?
Ans : Sulphuric acid is manufactured by the contact process. It involves the following steps:
Step (i):
Sulphur or sulphide ores are burnt in air to form SO2.
Step (ii):
By a reaction with oxygen, SO2 is converted into SO3 in the presence of V2O5 as a catalyst.
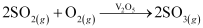
Step (iii):
SO3 produced is absorbed on H2SO4 to give H2S2O7 (oleum).
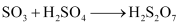
This oleum is then diluted to obtain H2SO4 of the desired concentration.
In practice, the plant is operated at 2 bar (pressure) and 720 K (temperature). The sulphuric acid thus obtained is 96-98% pure.