NEET-XII-Chemistry
05: Surface Chemistry
- Qstn #5What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Ans :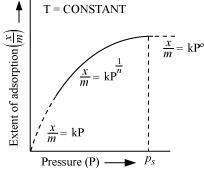
The plot between the extent of adsorption against the pressure of gas (P) at constant temperature (T) is called the adsorption isotherm.
against the pressure of gas (P) at constant temperature (T) is called the adsorption isotherm.
Freundlich adsorption isotherm:
Freundlich adsorption isotherm gives an empirical relationship between the quantity of gas adsorbed by the unit mass of solid adsorbent and pressure at a specific temperature.
From the given plot it is clear that at pressure PS,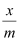 reaches the maximum valve. Ps is called the saturation pressure. Three cases arise from the graph now.
reaches the maximum valve. Ps is called the saturation pressure. Three cases arise from the graph now.
Case I- At low pressure:
The plot is straight and sloping, indicating that the pressure in directly proportional to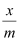 i.e.,
i.e., 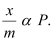

Case II- At high pressure:
When pressure exceeds the saturated pressure,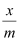 becomes independent of P values.
becomes independent of P values.
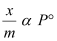
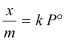
Case III- At intermediate pressure:
At intermediate pressure,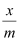 depends on P raised to the powers between 0 and 1. This relationship is known as the Freundlich adsorption isotherm.
depends on P raised to the powers between 0 and 1. This relationship is known as the Freundlich adsorption isotherm.
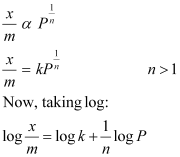
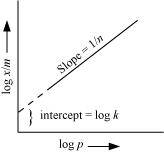
On plotting the graph between log and log P, a straight line is obtained with the slope equal to
and log P, a straight line is obtained with the slope equal to 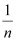 and the intercept equal to log k.
and the intercept equal to log k.
- Qstn #6What do you understand by activation of adsorbent? How is it achieved?
Ans : By activating an adsorbent, we tend to increase the adsorbing power of the adsorbent. Some ways to activate an adsorbent are:
(i) By increasing the surface area of the adsorbent. This can be done by breaking it into smaller pieces or powdering it.
(ii) Some specific treatments can also lead to the activation of the adsorbent. For example, wood charcoal is activated by heating it between 650 K and 1330 K in vacuum or air. It expels all the gases absorbed or adsorbed and thus, creates a space for adsorption of gases.
- Qstn #7What role does adsorption play in heterogeneous catalysis?
Ans : Heterogeneous catalysis:
A catalytic process in which the catalyst and the reactants are present in different phases is known as a heterogeneous catalysis. This heterogeneous catalytic action can be explained in terms of the adsorption theory. The mechanism of catalysis involves the following steps:
(i) Adsorption of reactant molecules on the catalyst surface.
(ii) Occurrence of a chemical reaction through the formation of an intermediate.
(iii) De-sorption of products from the catalyst surface
(iv) Diffusion of products away from the catalyst surface.
In this process, the reactants are usually present in the gaseous state and the catalyst is present in the solid state. Gaseous molecules are then adsorbed on the surface of the catalyst. As the concentration of reactants on the surface of the catalyst increases, the rate of reaction also increases. In such reactions, the products have very less affinity for the catalyst and are quickly desorbed, thereby making the surface free for other reactants.
- Qstn #8Why is adsorption always exothermic?
Ans : Adsorption is always exothermic. This statement can be explained in two ways.
(i) Adsorption leads to a decrease in the residual forces on the surface of the adsorbent. This causes a decrease in the surface energy of the adsorbent. Therefore, adsorption is always exothermic.
(ii) ΔH of adsorption is always negative. When a gas is adsorbed on a solid surface, its movement is restricted leading to a decrease in the entropy of the gas i.e., ΔS is negative. Now for a process to be spontaneous, ΔG should be negative.
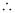 ΔG = ΔH - TΔS
ΔG = ΔH - TΔS
Since ΔS is negative, ΔH has to be negative to make ΔG negative. Hence, adsorption is always exothermic.
- Qstn #9How are the colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium?
Ans : One criterion for classifying colloids is the physical state of the dispersed phase and dispersion medium. Depending upon the type of the dispersed phase and dispersion medium (solid, liquid, or gas), there can be eight types of colloidal systems.
Dispersed phase
Dispersion medium
Type of colloid
Example
1.
Solid
Solid
Solid Sol
Gemstone
2.
Solid
Liquid
Sol
Paint
3.
Solid
Gas
Aerosol
Smoke
4.
Liquid
Solid
Gel
Cheese
5.
Liquid
Liquid
Emulsion
Milk
6.
Liquid
Gas
Aerosol
Fog
7.
Gas
Solid
Solid foam
Pumice stone
8.
Gas
Liquid
Foam
Froth
- Qstn #10Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Ans : Effect of pressure
Adsorption is a reversible process and is accompanied by a decrease in pressure. Therefore, adsorption increases with an increase in pressure.
Effect of temperature
Adsorption is an exothermic process. Thus, in accordance with Le-Chatelier’s principle, the magnitude of adsorption decreases with an increase in temperature.
- Qstn #11What are lyophilic and lyophobic sols? Give one example of each type. Why are hydrophobic sols easily coagulated?
Ans : (i) Lyophilic sols:
Colloidal sols that are formed by mixing substances such as gum, gelatin, starch, etc. with a suitable liquid (dispersion medium) are called lyophilic sols. These sols are reversible in nature i.e., if two constituents of the sol are separated by any means (such as evaporation), then the sol can be prepared again by simply mixing the dispersion medium with the dispersion phase and shaking the mixture.
(ii) Lyophobic sols:
When substances such as metals and their sulphides etc. are mixed with the dispersion medium, they do not form colloidal sols. Their colloidal sols can be prepared only by special methods. Such sols are called lyophobic sols. These sols are irreversible in nature. For example: sols of metals.
Now, the stability of hydrophilic sols depends on two things- the presence of a charge and the salvation of colloidal particles. On the other hand, the stability of hydrophobic sols is only because of the presence of a charge. Therefore, the latter are much less stable than the former. If the charge of hydrophobic sols is removed (by addition of electrolytes), then the particles present in them come closer and form aggregates, leading to precipitation.
- Qstn #12What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids?
Ans : (i) In multi-molecular colloids, the colloidal particles are an aggregate of atoms or small molecules with a diameter of less than 1 nm. The molecules in the aggregate are held together by van der Waal’s forces of attraction. Examples of such colloids include gold sol and sulphur sol.
(ii) In macro-molecular colloids, the colloidal particles are large molecules having colloidal dimensions. These particles have a high molecular mass. When these particles are dissolved in a liquid, sol is obtained. For example: starch, nylon, cellulose, etc.
(iii) Certain substances tend to behave like normal electrolytes at lower concentrations. However, at higher concentrations, these substances behave as colloidal solutions due to the formation of aggregated particles. Such colloids are called aggregated colloids.
- Qstn #13What are enzymes? Write in brief the mechanism of enzyme catalysis.
Ans : Enzymes are basically protein molecules of high molecular masses. These form colloidal solutions when dissolved in water. These are complex, nitrogenous organic compounds produced by living plants and animals. Enzymes are also called ‘biochemical catalysts’.
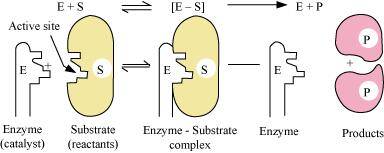
Mechanism of enzyme catalysis:
On the surface of the enzymes, various cavities are present with characteristic shapes. These cavities possess active groups such as -NH2, -COOH, etc. The reactant molecules having a complementary shape fit into the cavities just like a key fits into a lock. This leads to the formation of an activated complex. This complex then decomposes to give the product.
Hence,
Step 1: E + S → ES+
(Activated complex)
Step 2: ES+ → E + P
- Qstn #14How are colloids classified on the basis of
Ans : Colloids can be classified on various bases:
- Qstn #14-iPhysical states of componentsAns : On the basis of the physical state of the components (by components we mean the dispersed phase and dispersion medium). Depending on whether the components are solids, liquids, or gases, we can have eight types of colloids.
- Qstn #14-iiNature of dispersion medium andAns : On the basis of the dispersion medium, sols can be divided as:
-
Dispersion medium
Name of sol
Water
Aquasol or hydrosol
Alcohol
Alcosol
Benzene
Benzosol
Gases
Aerosol
-
- Qstn #14-iiiInteraction between dispersed phase and dispersion medium?Ans : On the basis of the nature of the interaction between the dispersed phase and dispersion medium, the colloids can be classified as lyophilic (solvent attracting) and lyophobic (solvent repelling).
- Qstn #15-iWhen a beam of light is passed through a colloidal sol.Ans : When a beam of light is passed through a colloidal solution, then scattering of light is observed. This is known as the Tyndall effect. This scattering of light illuminates the path of the beam in the colloidal solution.