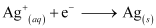NEET-XII-Chemistry
03: Electrochemistry
- #3-iWhich of the electrode is negatively charged?
() The carriers of the current in the cell.
() Individual reaction at each electrode.
() The carriers of the current in the cell.
() Individual reaction at each electrode.
() The carriers of the current in the cell.
() Individual reaction at each electrode.
() The carriers of the current in the cell.
() Individual reaction at each electrode.
() The carriers of the current in the cell.
() Individual reaction at each electrode.Ans : Zn electrode (anode) is negatively charged.
() Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
() The reaction taking place at the anode is given by,
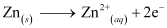
The reaction taking place at the cathode is given by,
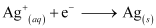
() Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
() The reaction taking place at the anode is given by,
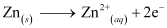
The reaction taking place at the cathode is given by,
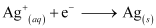
() Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
() The reaction taking place at the anode is given by,
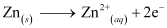
The reaction taking place at the cathode is given by,
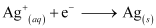
() Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
() The reaction taking place at the anode is given by,
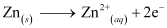
The reaction taking place at the cathode is given by,
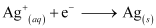
() Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
() The reaction taking place at the anode is given by,
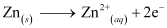
The reaction taking place at the cathode is given by,
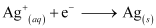
- #3-iiThe carriers of the current in the cell.Ans : Ions are carriers of current in the cell and in the external circuit, current will flow from silver to zinc.
- #3-iiiIndividual reaction at each electrode.Ans : The reaction taking place at the anode is given by,
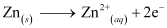
The reaction taking place at the cathode is given by,