NEET-XII-Chemistry
02: Solutions
- #36100
g of liquid A (molar mass 140 g mol-1)
was dissolved in 1000 g of liquid B (molar mass 180 g mol-1).
The vapour pressure of pure liquid B was found to be 500 torr.
Calculate the vapour pressure of pure liquid A and its vapour
pressure in the solution if the total vapour pressure of the solution
is 475 Torr.Ans : Number of moles of
liquid A,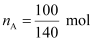
= 0.714 mol
Number of moles of
liquid B,
= 5.556 mol
Then, mole fraction of
A,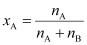
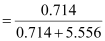
= 0.114
And, mole fraction of
B, xB = 1 - 0.114
= 0.886
Vapour pressure of pure
liquid B, =
=
500 torr
Therefore, vapour
pressure of liquid B in the solution,

= 500 × 0.886
= 443 torr
Total vapour pressure
of the solution, ptotal = 475 torr
 Vapour
Vapour
pressure of liquid A in the solution,
pA = ptotal - pB
= 475 - 443
= 32 torr
Now,


= 280.7 torr
Hence, the vapour
pressure of pure liquid A is 280.7 torr.
Page No 62: