NEET-XII-Chemistry
02: Solutions
- #25Amongst the following compounds, identify which are insoluble, partially
soluble and highly soluble in water?
(i) phenol (ii) toluene (iii) formic acid
(iv) ethylene
glycol (v) chloroform (vi) pentanol.Ans : (i) Phenol (C6H5OH) has the polar group
-OH and non-polar group -C6H5.
Thus, phenol is partially soluble in water.
(ii) Toluene
(C6H5-CH3) has no polar
groups. Thus, toluene is insoluble in water.
(iii) Formic acid (HCOOH) has the polar group -OH and
can form H-bond with water. Thus, formic acid is highly soluble in
water.
(iv) Ethylene glycol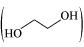 has polar -OH group and can form H-bond. Thus, it is
has polar -OH group and can form H-bond. Thus, it is
highly soluble in water.
(v) Chloroform
is insoluble in water.
(vi) Pentanol (C5H11OH) has polar -OH
group, but it also contains a very bulky non-polar -C5H11 group. Thus, pentanol is partially soluble in water.