NEET-XII-Chemistry
02: Solutions
- #13The partial pressure of ethane over a solution containing 6.56 × 10-3 g of ethane is 1 bar. If the solution contains 5.00 × 10-2 g of ethane, then what shall be the partial pressure of the gas?Ans : Molar mass of ethane (C2H6) = 2 × 12 + 6 × 1
= 30 g mol-1
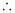 Number of moles present in 6.56 × 10-3 g of ethane
Number of moles present in 6.56 × 10-3 g of ethane
= 2.187 × 10-4 mol
Let the number of moles of the solvent be x.
According to Henry’s law,
p = KHx

Number of moles present in 5.00 × 10-2 g of ethane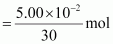
= 1.67 × 10-3 mol
According to Henry’s law,
p = KHx

= 7.636 bar
Hence, partial pressure of the gas shall be 7.636 bar.