Loading…
NEET-XII-Chemistry
02: Solutions
- #11Why
do gases always tend to be less soluble in liquids as the temperature
is raised?Ans : Solubility of gases in
liquids decreases with an increase in temperature. This is because
dissolution of gases in liquids is an exothermic process.
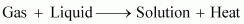
Therefore, when the
temperature is increased, heat is supplied and the equilibrium shifts
backwards, thereby decreasing the solubility of gases.