NEET-XII-Chemistry
02: Solutions
- #14What
is meant by positive and negative deviations from Raoult’s law and
how is the sign of ΔsolH related to positive and negative deviations from Raoult’s law?Ans : According
to Raoult’s law, the partial vapour pressure of each volatile
component in any solution is directly proportional to its mole
fraction. The solutions which obey Raoult’s law over the entire
range of concentration are known as ideal solutions. The
solutions that do not obey Raoult’s law (non-ideal solutions)
have vapour pressures either higher or lower than that predicted by
Raoult’s law. If the vapour pressure is higher, then the
solution is said to exhibit positive deviation, and if it is lower,
then the solution is said to exhibit negative deviation from Raoult’s
law.
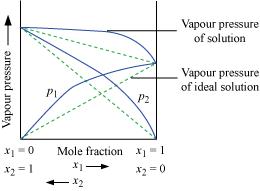
Vapour
pressure of a two-component solution showing positive
deviation from Raoult’s law
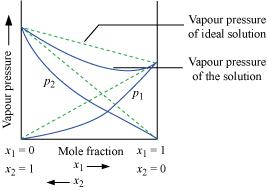
Vapour pressure of a two-component solution showing negative
deviation from Raoult’s law
In the
case of an ideal solution, the enthalpy of the mixing of the pure
components for forming the solution is zero.
ΔsolH = 0
In the
case of solutions showing positive deviations, absorption of heat
takes place.
∴ΔsolH = Positive
In the
case of solutions showing negative deviations, evolution of heat
takes place.
∴ΔsolH = Negative