NEET-XII-Chemistry
02: Solutions
- #7Henry’s
law constant for CO2 in water is 1.67 × 108 Pa at 298 K. Calculate the quantity of CO2 in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.Ans : It
is given that:
KH = 1.67 × 108 Pa
 = 2.5 atm = 2.5 × 1.01325 × 105 Pa
= 2.5 atm = 2.5 × 1.01325 × 105 Pa
=
2.533125 × 105 Pa
According
to Henry’s law:
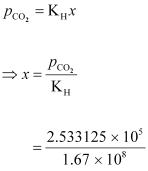
= 0.00152
We
can write,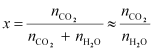
[Since, is
is
negligible as compared to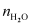 ]
]
In
500 mL of soda water, the volume of water =
500 mL
[Neglecting
the amount of soda present]
We can
write:
500 mL of
water = 500 g of water
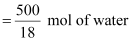
= 27.78 mol of water
Now,
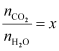
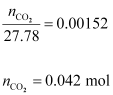
Hence,
quantity of CO2 in 500 mL of soda water = (0.042 × 44)g
= 1.848 g
Page No 47: