ICSE-X-Chemistry
Previous Year Paper year:2010
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #1-c-ixix. The organic compound mixed with ethanol to make it spurious is
(A) Methanol
(B) Methanoic acid
(C) Methanol
(D) Ethanoic acidAns : (A)
- #1-c-vv. During ionisation, metals lose electrons; this change can be called
(A) Oxidation
(B) Reduction
(C) Redox
(D) DisplacementAns : (A)
- #1-c-vivi. Which one of the following is not true of metals?
(A) Metals are good conductors of electricity.
(B) Metals are malleable and ductile.
(C) Metals form non-polar covalent compounds.
(D) Metals will have 1 or 2 or 3 electrons in their valence shell.Ans : (C)
- #1-c-viivii. An example of a complex salt is
(A) Zinc sulphate
(B) Sodium hydrogen sulphate
(C) Iron (II) ammonium sulphate
(D) Tetrammine copper (II) sulphateAns : (D)
- #1-c-viiiviii. Aqua regia is a mixture of
(A) Dilute hydrochloric acid and concentrated nitric acid
(B) Concentrated hydrochloric acid and dilute nitric acid
(C) Concentrated hydrochloric acid (1 part) and concentrated nitric acid (3 parts)
(D) Concentrated hydrochloric acid(3 parts) and concentrated nitric acid(1 part)Ans : (D)
- #1-c-xx. The number of electrons present in the valence shell of a halogen is
(A) 1
(B) 3
(C) 5
(D) 7Ans : (D)
- #1-d [5]State your observation for the following cases:
- #1-d-ii. Moist blue litmus is introduced into a gas jar of sulphur dioxide.Ans : out of current syllabus
- #1-d-iiii. Dry red rose petals are placed in the jar of sulphur dioxide.Ans : out of current syllabus
- #1-d-iiiiii. Paper soaked in potassium permanganate solution is introduced into a gas jar of
sulphur dioxide.Ans : out of current syllabus
- #1-d-iviv. Ammonia gas is burnt in an atmosphere of oxygen in the absence of a catalyst.Ans : It burns with a yellowish green flame producing water vapour and nitrogen.
- #1-d-vv. A glass rod dipped in ammonium hydroxide is brought near the mouth of the
concentrated hydrochloric acid bottle.Ans : It produce dense white fumes of ammonium chloride.
- #1-e [5]Match the Column A with Column B:
Column A Column B (i) Sodium chloride Increases (ii) Ammonium ion Covalent bond (iii) Electronegativity across the period Ionic bond (iv) Non-metallic character down the group Covalent and coordinate bond (v) Carbon tetrachloride Decreases
Answer as follows:
(i.) Correct item from B matching sodium chloride
(ii.) Correct item from B matching ammonium ion and so on.Ans :
(i) Sodium chloride - Ionic bond.
(ii) Ammonium ion - Covalent and Co-ordinate bond.
(iii) Electronegativity across a period - Increases.
(iv) Non metallic character down the group - Decreases.
(v) Carbon tetrachloride - Covalent bond.
- #1-f [5]Write the equation for each of the following reactions:Ans :
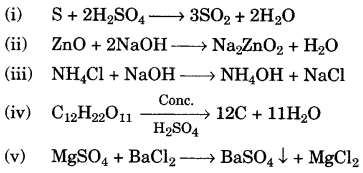
- #1-f-ii. Sulphur is heated with concentrated sulphuric acid.