ICSE-X-Chemistry
Previous Year Paper year:2019
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- #2-c-iiThe ion of which element will migrate towards the cathode during electrolysis?Ans : A [Positive ions migrate towards cathode, hence A will form A+ and migrate towards cathode]
- #2-c-iiiWhich non-metallic element has the valency of 2?Ans : E
- #2-c-ivWhich is an inert gas?Ans : F
- #3
- #3-aName the particles present in:
- #3-a-iStrong electrolyteAns : Only ions
- #3-a-iiNon-electrolyteAns : molecules
- #3-a-iiiWeak electrolyteAns : Ions as well as molecules
- #3-bDistinguish between the following pairs of compounds using the reagent given in the bracket.
[3]
- #3-b-iManganese dioxide and copper (II) oxide, (using concentrated HCl)
Ans :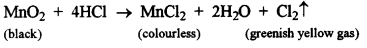
- #3-b-iiFerrous sulphate solution-and ferric sulphate solution, (using sodium hydroxide solution)
Ans :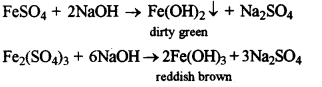
- #3-b-iiiDilute hydrochloric acid and dilute sulphuric acid, (using lead nitrate solution)
Ans :
- #3-cChoose the method of preparation of the following salts, from the methods given in the list:
[4]
[List: A. Neutralization
B. Precipitation
C. Direct combination
D. Substitution]
- #3-c-iLead chlorideAns : Lead chloride → Precipitation(B)
- #3-c-iiIron (II) sulphateAns : Iron (II) sulphate → Substitution (D)