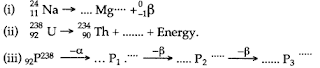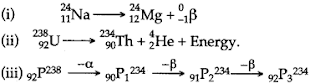ICSE-X-Physics
12: Radioactivity
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #ICSE Answers for Chapter 12 Radioactivity Class 10 Selina
- #Section : AExercise 12 A
- Qstn #1What do you understand by the term radioactivity?Ans : 1 The process of self, spontaneous and random emission of a or P and y radiations from the nucleus of elements of atomic number higher than 82, is called radioactivity.
- Qstn #2What are radioactive substances? Give two examples of such substances.Ans : 2 Radioactive substances are those which have the property of self emission of α or β and γ radiations.
Examples: Uranium, Thorium.
- Qstn #3Name the different radiations which are emitted by the radioactive substances.
Are all the radiations mentioned by you, emitted in a single radioactive decay?Ans : 3 Following three radiations are emitted by the radioactive substances:
(i) Alpha (α), (ii) Beta (β), and (iii) Gamma (γ).
All the above radiations are not emitted in a single radioactive decay. There will be either an α emission or a β emission, which may be accompained by γ-emission.
- Qstn #4Are all the radiations viz. α, β and γ emitted in a single radioactive decay?Ans : 4 All the above radiations are not emitted in a single decay. There will be either an α emission or a β emission, which may be accompanied by γ-emission.
- Qstn #5Compare the penetrating powers of α, β and γ-radiations.Ans : 5 γ -radiations are nearly 100 times more penetrating than β-radiations and 10,000 times more penetrating than α-radiations.
- Qstn #6Compare the ionising powers of α, β and γ radiations.Ans : 6 The ionising power of α-radiation is nearly 100 times that of β radiations and nearly 10,000 times that of γ-radiations.
- Qstn #7State the kind of nuclear reaction taking place in a nucleus during the emission of a β-particle.Ans : 7 In the process of emission of a β-particle from the nucleus, a neutron changes into a proton by emitting a β-particle (electron).
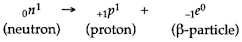
Hence in the nucleus, the number of neutrons decreases by 1 with the emission of a β-particle and the number of protons increases by one. So the sum of protons and neutrons, i.e., atomic mass remains the same but the atomic number increases by one.
- Qstn #8A certain nucleus has a mass number 20 and atomic number 9. Find the number of neutrons and protons present in it.Ans : 8 (i) Number of neutrons = Mass number - Atomic number
= 20 - 9 = 11
(ii) Number of protons = Atomic number = 9.
- Qstn #9Justify with reasons, whether the following nuclear reactions are allowed or not.
 Ans : 9 (i) This reaction is allowed because, the mass number as well as atomic number are conserved here.
Ans : 9 (i) This reaction is allowed because, the mass number as well as atomic number are conserved here.
(ii) This reaction is not allowed because even though charge (atomic number) is conserved in it, the mass number [= A on the L H.S. and equal to (A + 4)] on the R.H.S. is not conserved.
- Qstn #10An electrons emitter must have how much work function and melting point.Ans : 10 An electron emitter must have low work function and high melting point.
- Qstn #11A radioactive nucleus undergoes a series of decays according to the sequence
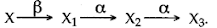
If the mass number and atomic number of X3 are 172 and 69 respectively, what is the mass number and atomic number of X?Ans : 11 Mass no. of X = 180
Atomic no. of X = 72
- Qstn #12A mixture of radioactive substances gives off three types of radiations: (i) Name the radiation which travels with the speed of light. (ii) Name the radiation which has the highest ionizing power.Ans : 12 (i) γ-rays.
(ii) α-particles.